How Softeners Work
Most water softeners on the market are ion exchange systems. In these systems, hardness ions (calcium and magnesium) are exchanged for salt (sodium or potassium) ions. The exchange takes place within the resin tank of the water softener. When water flows through the tank it comes in contact with small resin beads that are covered with salt ions. As water flows through the resin beads, hardness ions trade places with salt ions, hence the higher salt content in softened water.
As larger volumes of water are softened, the beads become exhausted and contain nothing but hardness ions . The beads are recharged by adding bags of salt to the brine tank. Recharging works the same as softening but in reverse. The hardness ions swap places with the salt ions in the brine tank and excess minerals are rinsed into the wastewater drain.
Demand initiated regeneration (DIR) water softeners are the most common ion exchange softeners sold locally. DIR softeners meter water usage over time and only regenerate when needed. Some softeners operate on a timer or schedule that regenerates at set increments. This older technology can be very wasteful of both salt and water because the softener will regenerate even during periods of low household water use (e.g. vacation away from home). At the same time, these models can leave you short of soft water if you have periods of higher water use (e.g. house guests).
Read our FAQ about the different types of water softeners available
Did you know:
Although rock salt is cheaper than solar or evaporated salts, it contains more insoluble material. Solar and evaporated salts keep your water softener cleaner.
Water softener components
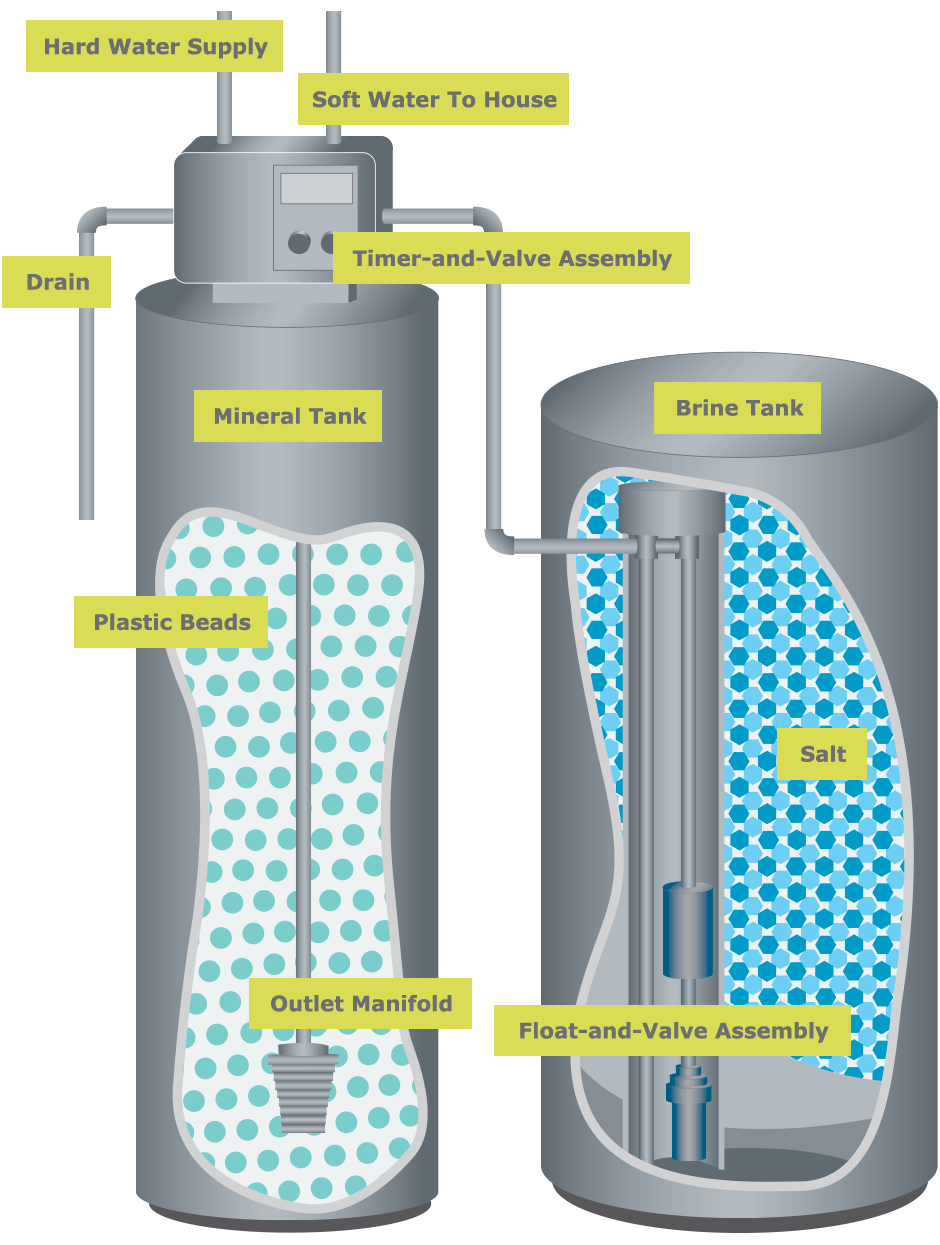
Water softener components

What are the differences between water softener salts?
There are generally three different types of water softener salts available:
- Rock salt
- Solar salt – Salt produced by the evaporation of seawater
- Evaporated salt – Refined and completely purified salt, such as table salt
Rock salt is cheaper than solar or evaporated salts but contains more insoluble material. As a result, solar and evaporated salts keep your water softener cleaner. The type of salt you choose to buy will depend on the frequency of regeneration. If your water softener regenerates a lot you’ll want to use solar or evaporated salts to avoid the fast build-up of non-soluble matter from rock salt. For the average home owner this should not be a concern as excess minerals are rinsed away during regeneration.
For those concerned about the sodium content in their drinking water, potassium chloride is an alternative to sodium chloride. Potassium chloride is slightly more expensive to buy but works in the same way as regular water softening salt. Potassium chloride is safe for most water softeners but consult your owner’s manual before switching.
Are there alternative technologies for treating hard water?
Some retailers sell alternatives to traditional water softeners that prevent scale buildup without the use of salt, and sometimes even without water. Most of these alternative technologies don’t soften water—i.e. they don’t remove calcium and magnesium. Instead, these alternatives change the properties of suspended solids—small, solid particles suspended in water like calcium and magnesium—to stop scale from forming on water heater elements, taps and showerheads, clothes and dish washers, and other water using appliances. Examples of these alternatives include:
- Electrically induced precipitation
- Electromagnetic water treatment
- Capacitive Deionization (CDI)
- Template Assisted Crystallization (TAC) or Nucleation Assisted Crystallization (NAC)
A recent study published by the U.S. Water Reuse Research Foundation (WRF) clarifies the effectiveness of the four alternatives listed above. Click here to read the study.
The WRF study concluded that all four alternatives prevented scale buildup on water heater elements with varied results. NAC/TAC reduced scale buildup on water heater elements by about 90 per cent, matching the effectiveness of salt-based water softeners. The NAC/TAC units needed no salt or water to operate.
How it works
The NAC/TAC units use polymeric beads with nucleation sites to convert dissolved hardness into microscopic crystals. These crystals are then released by the beads (or “media”) and remain as insoluble particles that will not form scale on surfaces. No salt, water, or energy is required. Figure 1 below illustrates the crystallization process.
Figure 1 – Nucleation Assisted Crystallization / Template Assisted Crystallization
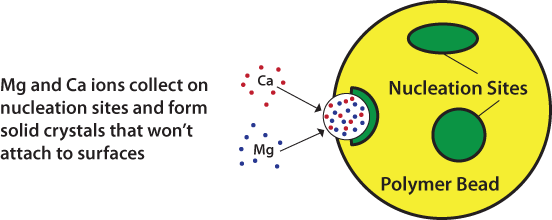
How much do they cost?
The 2015 purchase prices for NAC/TAC units tested by the Region of Waterloo and City of Guelph were comparable to purchase prices for equivalent ion exchange (salt-based) water softeners (estimated range $999–$1,499). The estimated price to replace exhausted NAC/TAC media every four years ranges from $250 to $875, which is $62 to $218 on an annual basis. This compares favourably with the estimated annual operating costs of ion exchange water softening of $125 to $248 per year per household for salt, water and energy (Region of Waterloo Residential Water Softener Performance Study Testing Report, October 5, 2012).
What’s the advantage?
With similar costs, the advantage to NAC/TAC units comes largely in reduced care and maintenance as the media only needs to be replaced about every four years. There are also environmental benefits from using less water, salt and energy, and health benefits for those without hard water taps for drinking.
Known manufacturers (as of December 2015)
- Watts Water Technologies
- Next Filtration Technologies
- WaterGroup
- Pelican Water Systems
- Clearwater Solutions
- Excalibur Water Systems
- Aquasana
NAC/TAC study results
Between 2017 and 2018, Region of Waterloo and City of Guelph hired Metroline Research Group to complete a market research study that involved installing salt-free units in 18 volunteer households. The goals were to see how well they performed in real world conditions and how people felt about them after a year of use.
Some key findings of the study include:
- Most people who tried the technology were satisfied. In the end, 13 of 18 participants decided to keep and continue to use their salt-free units.
- The biggest benefits people saw after switching to salt-free conditioners were:
- freedom from having to buy salt, lug it into the house and remembering to load it
- saving money on salt, water and electricity bills
- reducing their environmental impact
- cleaning out calcium build up in the home’s plumbing
- ability to drink water from any tap without worrying about salt content
- The biggest drawbacks people who did not keep their units saw were:
- concern that their dishwashers left behind too much film or residue on dishes and cutlery, meaning extra wiping and cleaning
- uncertainty about whether the system was working properly or if the beads they use to condition water need replacing
- a general feeling, for at least some people in the house, that the new technology does not perform as well.
- Salt-free water conditioners are not for everyone. When deciding how to deal with hard water in your home consider what kinds of trade offs you are willing to make in cost, performance and lifestyle.
For more details read Are salt-free water conditioning systems right for you? A study of consumer preferences, or the full report: A Salt-free Alternative to Residential Water Softeners: Market Research Study (2019).
During 2014 and 2015, the Region of Waterloo and City of Guelph completed a market study and performance test of NAC/TAC. Here are some quick findings from the partnership:
- The technology was developed in Germany 20 years ago and is widely accepted for commercial and residential use throughout Europe.
- Some units have been certified by the German Technical and Scientific Institute for Gas and Water (DVGW).
- The technology was licensed for use in North America in 2002 and has passed NSF health/safety standards.
- NAC/TAC technology performed the way it was supposed to during limited testing by the Region of Waterloo and City of Guelph
- The Region of Waterloo and City of Guelph test results suggest NAC/TAC media be replaced every four years to remain effective; check with suppliers to confirm.
For more details read the NAC/TAC study report by Dr. Peter Fox.
